Exploring Low-Cost Adsorbents for Efficient Fluoride Removal
##plugins.themes.academic_pro.article.main##
Abstract
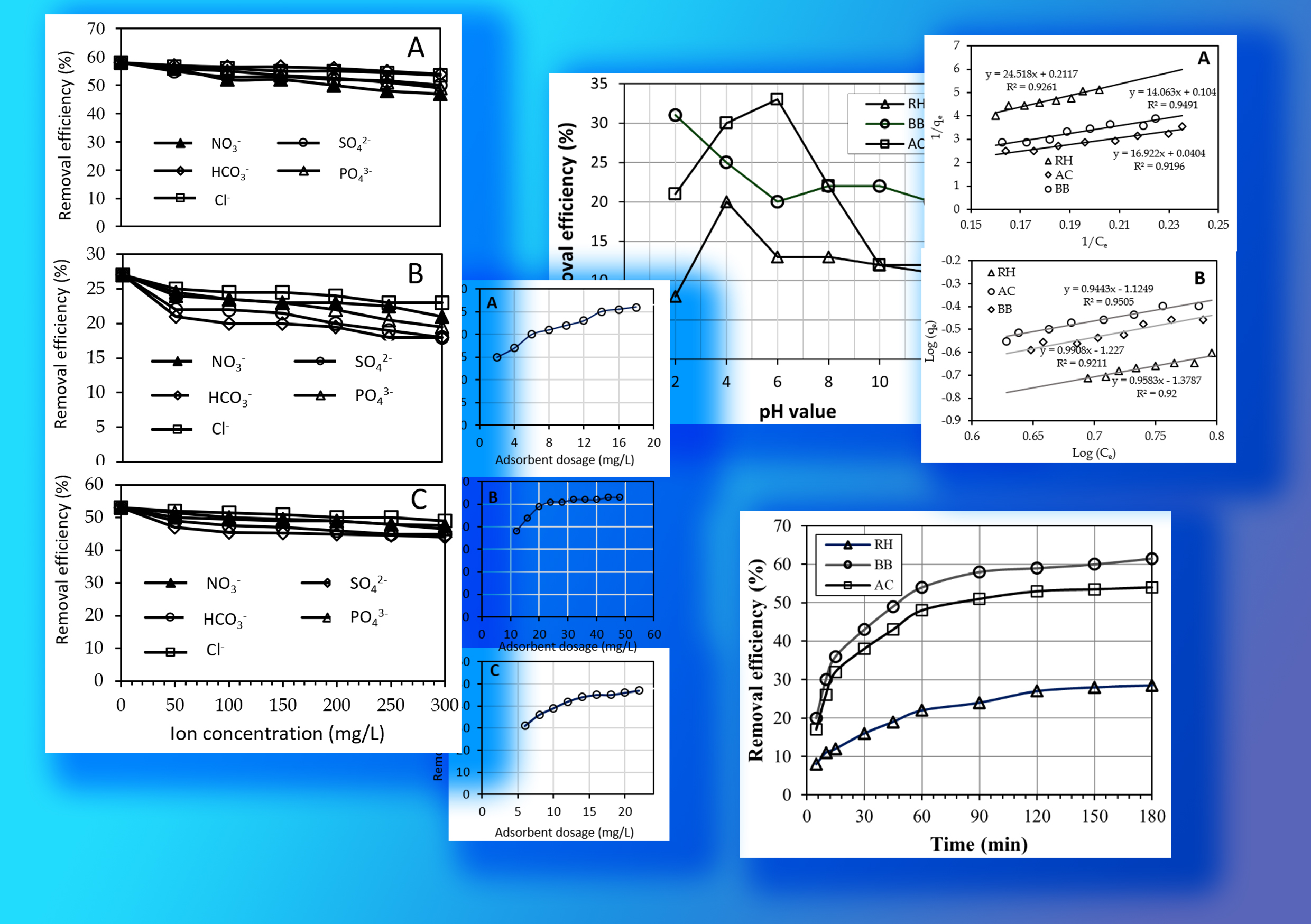
Fluoride contamination in water poses a significant health risk, emphasizing the need for effective removal methods. This study examines the efficacy of low-cost adsorbents: Rice Husk (RH), Broken bricks (BB), and Activated Carbon (AC)—for fluoride removal. Batch experiments were conducted to characterize these adsorbents and evaluate their performance across varying pH levels. Results reveal a substantial impact of pH on fluoride removal efficiency, with RH, BB, and AC exhibiting optimal rates at pH 4, 2, and 6, respectively. This underscores the influence of pH-dependent surface charge characteristics and fluoride ion speciation on removal efficiencies. The results of competition studies revealed that the adsorbent BB demonstrates the highest affinity for fluoride ions compared to RH and AC suggesting that BB is more effective at adsorbing fluoride ions compared to the other adsorbents. Furthermore, analysis of adsorption kinetics and optimal dosages uncovers differences in adsorption mechanisms among the adsorbents. RH adheres to the Langmuir model, while BB follows the Freundlich model, and AC outperforms according to both models. These findings underscore variations in adsorption capacities, affinities, and mechanisms, with AC emerging as a promising option for efficient fluoride removal. Nonetheless, further research is necessary to fully comprehend the nuanced mechanisms behind pH-dependent fluoride removal and to explore potential pre-treatment strategies to enhance RH and BB's adsorption capacity. In summary, this study provides valuable insights into developing efficient fluoride removal strategies using economical adsorbents.