Exhaustive Grignard Ethylation on N-benzylcinchomeronic Imide and Investigating Its Outcomes
##plugins.themes.academic_pro.article.main##
Abstract
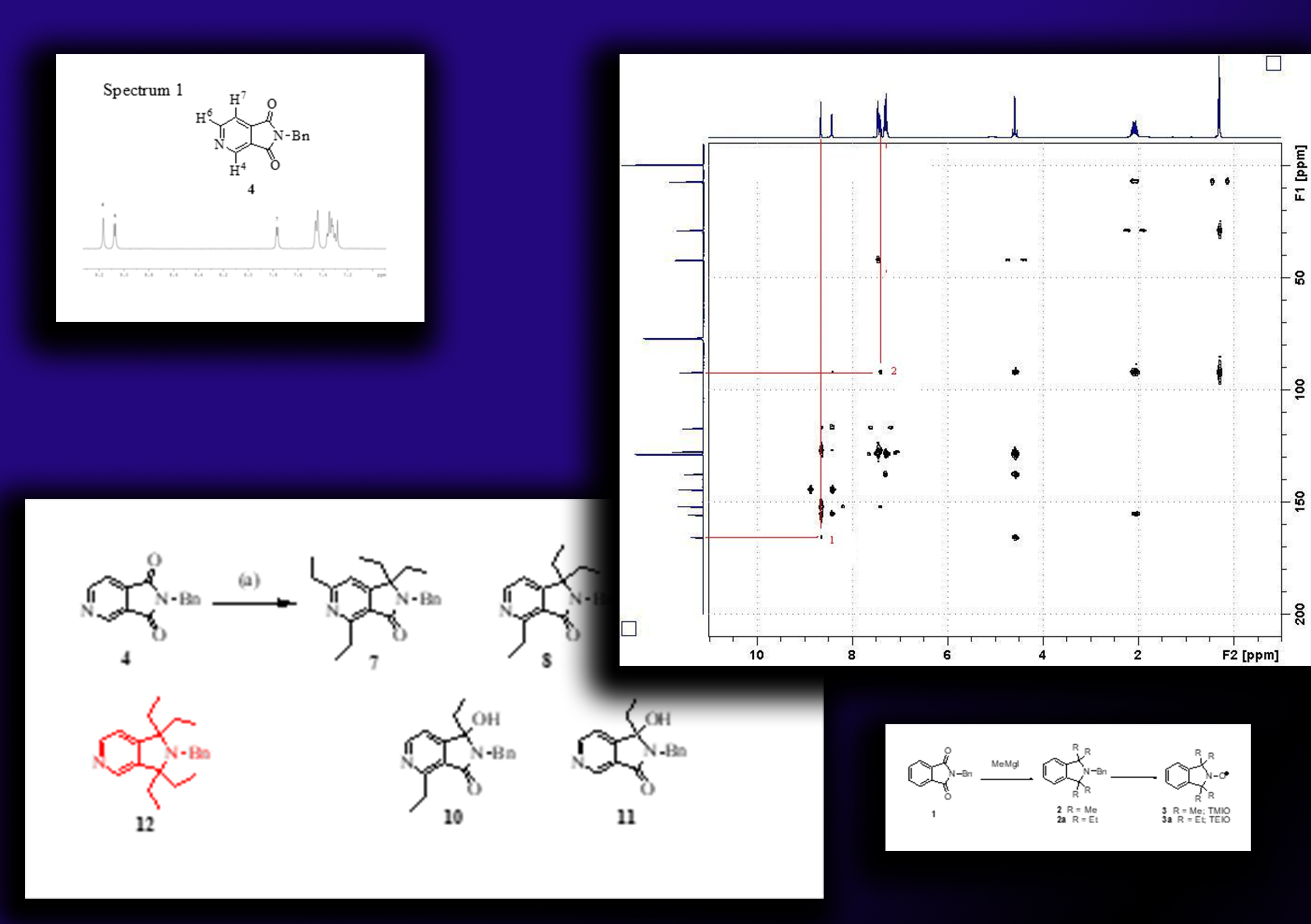
The progress of the Grignard tetraethylation of N-benzylcinchomeronic imide, a crucial step in the synthesis of the desired tetraethyl version of pyridine-annulated pyrrolidine nitroxide, was investigated in this study to obtain an insight of this reaction. Previous knowledge gathered during the tetraethylation of isoindoline systems was utilized in this study. During the investigation, it was found that the synthesis of the tetraethyl-pyridine-adduct was not achievable due to the formation of several side products such as 1,1-diethyl adducts and some ring substituted products, which are unlikely to be intermediates on the pathway to form the expected tetraethyl adduct.
##plugins.themes.academic_pro.article.details##
How to Cite
Viraj C. Jayawardena. (2023). Exhaustive Grignard Ethylation on N-benzylcinchomeronic Imide and Investigating Its Outcomes. Sri Lankan Journal of Applied Sciences, 1(02), 50–56. Retrieved from https://sljoas.uwu.ac.lk/index.php/sljoas/article/view/56