Investigation of Lipase Producing Bacteria from Oil Contaminated Soil and Characterization of Lipase
##plugins.themes.academic_pro.article.main##
Abstract
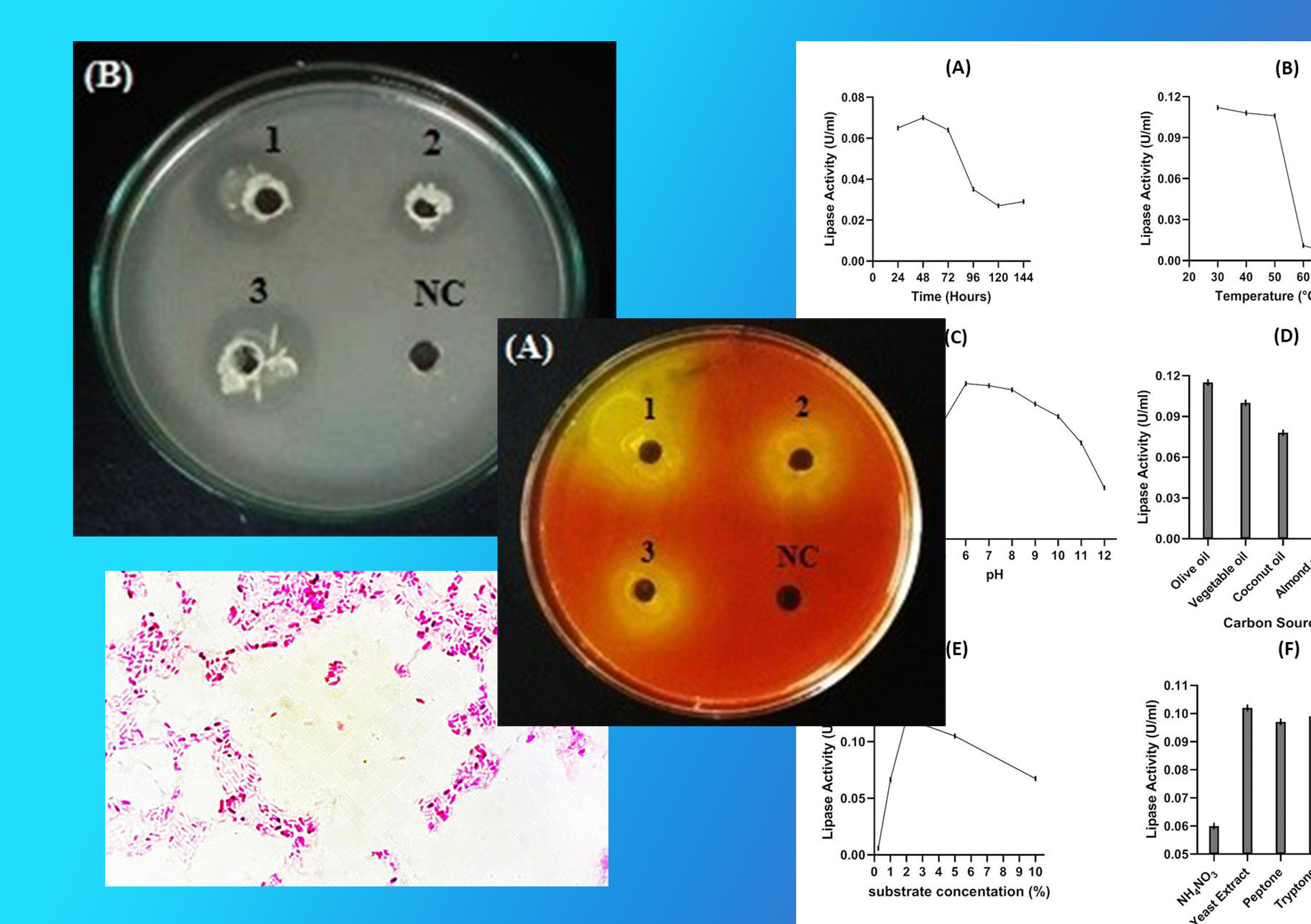
Hydrolytic enzymes such as lipases have emerged as key enzymes in a broad array of biotechnological industries due to their multifaceted characteristics. Many of the lipases that are currently in use in industry are of microbial origin. The aim of the present study was to isolate and identify lipase producing bacteria from oil contaminated soil and subsequent optimization of their culture conditions to maximize lipase production. Lipase producing bacterium Burkholderia sp. was isolated and identified by morphological studies, biochemical methods and 16S rDNA sequencing method. pH, incubation period, temperature, carbon source, nitrogen source and substrate concentration were studied to determine the optimum culture conditions for enzyme production. Media optimization studies showed that culture condition and media composition should be at pH 6, 30 °C, 48 hours of culture in a medium containing 2% olive oil as the main carbon source and yeast extract as the main nitrogen sources to maximize the production of lipase of Burkholderia sp. The crude enzyme exhibited hydrolytic activity in a wide range of temperatures (30 – 50 °C) and pH values (6–12), with an optimal temperature at 40° C and optimal pH at 8 with para-nitrophenyl palmitate as the substrate. Metal ions such as Cu2+, Mn2+ and Zn2+ inhibited lipase activity. The enzyme preferably acted on olive oil as a substrate. The results are a clear indication that Burkholderia sp. has a capacity to produce lipases and can be considered as a potential candidature for biotechnological applications.
Keywords: 16S rDNA sequencing, Burkholderia sp., Lipases, pNPP assay