The comparison of ion interactions in sodium and magnesium based ionic liquid electrolytes with Al2O3 nano-particles Abstract Section Science and Technology
##plugins.themes.academic_pro.article.main##
Abstract
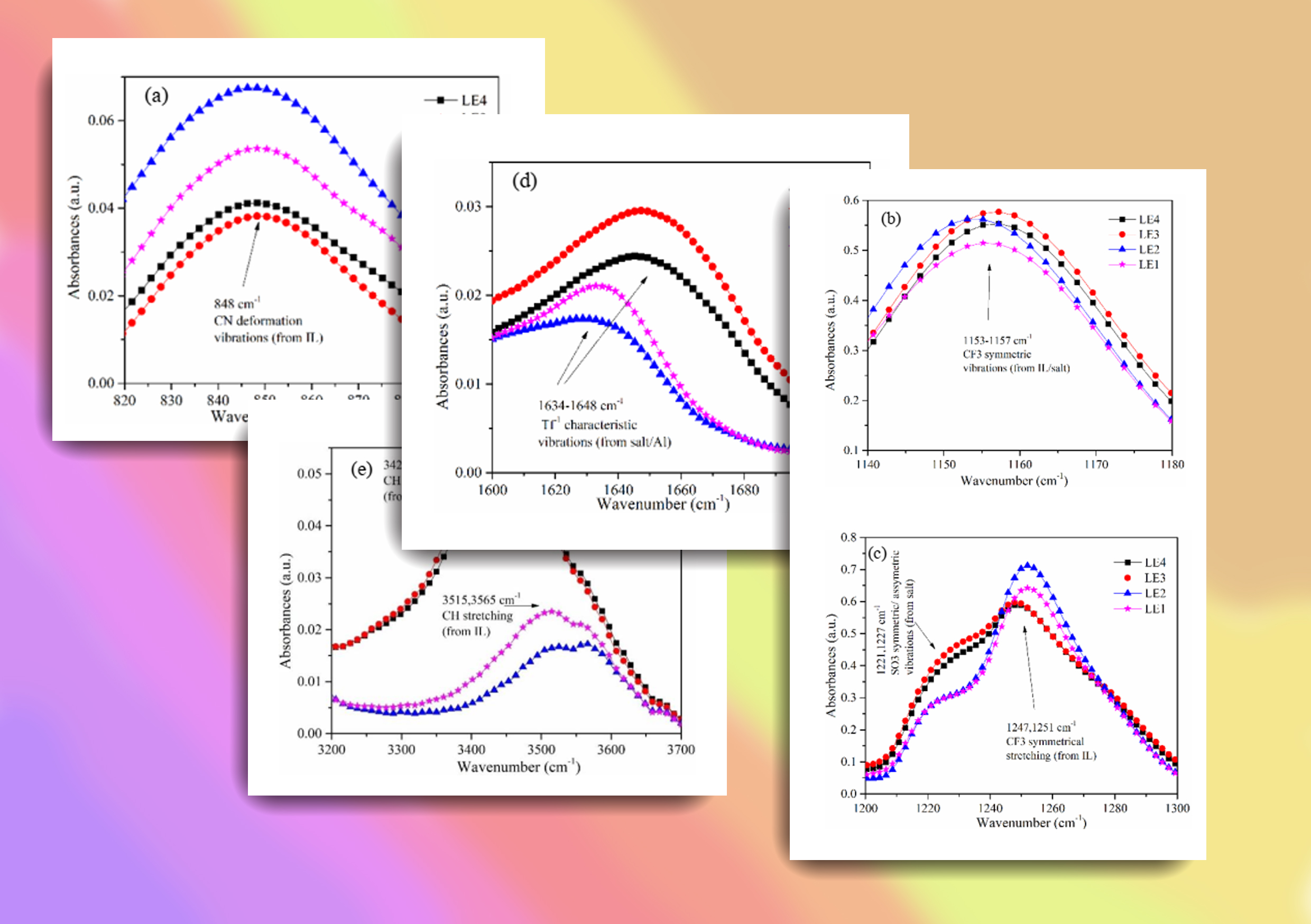
Ionic liquid (ILs) based electrolytes are novel and considered as safer electrolytes for future rechargeable batteries due to their non-volatility and non-flammability. Lithium salts doped ILs have been studied up to a great extent, however, studies on incorporation of other salts based on sodium (Na) or magnesium (Mg) into ILs are lacking. Since ion transport properties such as ionic conductivity of these IL-salt mixtures are directly depended on the molecular structure and interactions, spectroscopic studies will be helped to develop these materials. In this work, we explore the ionic interactions of sodium triflate (NaTf) and magnesium triflate (MgTf) doped IL systems based on 1-butyl-3-methylimidazolium trifluromethanesulfonate (BMIMTf) and alumina (Al2O3) nano-filler by FTIR spectroscopy. The 10 wt% of Al2O3 nano-filller was added to each IL-salt mixture to investigate the change of solvation structure. Results clearly showed that, the addition of Al2O3 nanoparticles indicates clear influence in the Na electrolyte system but weak in the Mg electrolyte system. This indicates that the Al2O3 nano-filler is more favored to interact with monovalent cation (Na+) rather than divalent cation (Mg2+). These findings will help to understand the behavior of IL based liquid electrolyte with nanofiller and guide experimentalists in optimizing IL-based electrolyte materials.